
QUALITY
We are an ISO 9001:2008, NP EN ISO 9001:2015, EN ISO 13485:2012(2016) and CE Marking no 0051 certified manufacturer of medical devices, that meets the highest costumer and dentistry market statutory and regulatory requirements. In CROSSBAUM®, quality is our main concern and our Management Quality System is subject to regular internal and external audits, that matches the high quality and prosthetic solutions required by the market.
Quality & Technology
WARRANTY
BIOLOGICAL TESTS
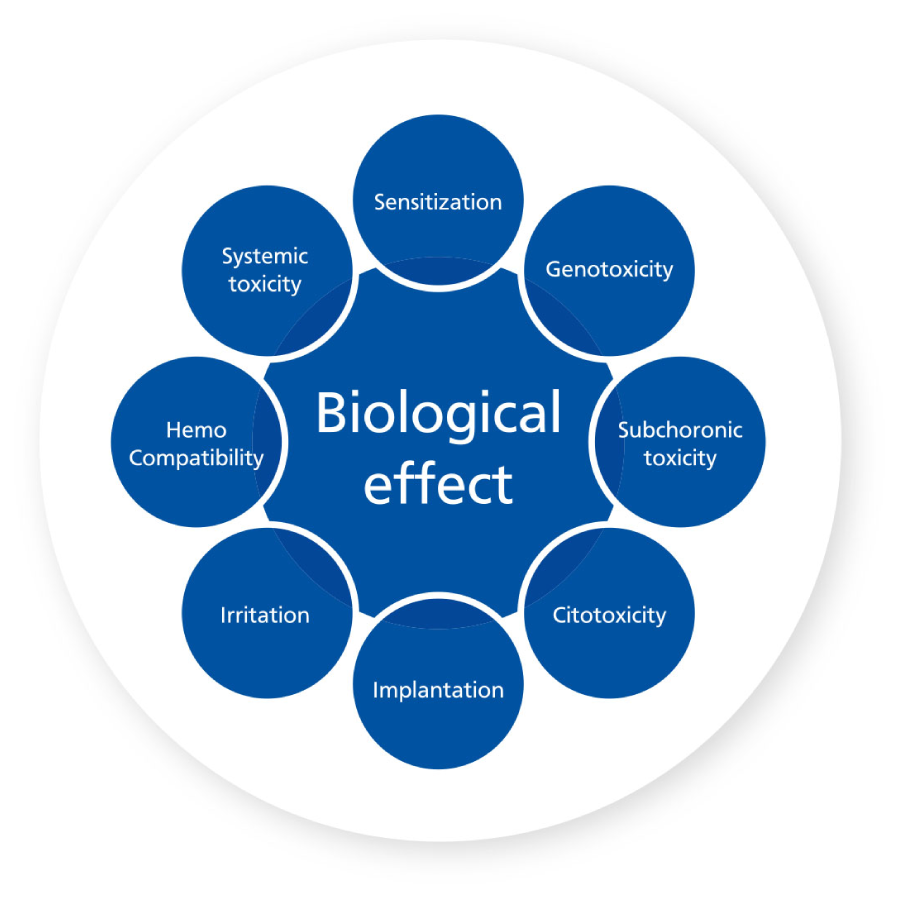
In CROSSBAUM®, all products have been evaluated for biological safety, confirming the biocompatibility of the titanium used to manufacture the devices and to support the evaluation of biological safety from the start.
It is of the upmost importance to develop a process of biological evaluation, in order to determine the performance of the prosthetic components for implants, the selection of material and the characterization and verification of the
biological safety.

STERILIZATION
CROSSBAUM® prosthetic components for implants are Non-Steril, but nevertheless, we recommend the parameters for an efficient cleaning and sterilization that were subject to validations tests performed by an external laboratory of international recognized merit. Vapor sterilization is the preferred and recommended sterilization method with the following parameters (T≥ 135° C, ≥ 5min), with a drying period of 10 min and a pressure between 1 and 3 bar. However, do not sterilize the titanium components in the original bags, since they are not compatibles with the recommended sterilization method.
MECHANICAL TESTS
CROSSBAUM® prosthetic components for implants were subject to mechanical tests, namely Bending Fatigue Test under the standard ISO 14801:2016 and the Torsion Test under the standard ISO 13498:2011, in order o guarantee the intended purpose of the prosthetic components as well its safety use.

CONTACT A SPECIALIST
Get immediate advice on products tailored to your needs.
